What Is Clean Steam?
Pharmaceutical settings need to maintain excellent standards of cleanliness for equipment, process systems, and the environment. Clean steam is a sterile, safe steam that can be used to sterilize and clean items in a cleanroom or other pharmaceutical or medical setting with strict cleanliness criteria. The industry uses clean steam—rather than standard steam—for higher levels of steam sterilization and humidification.
How Is Clean Steam Used in Pharmaceutical Settings?
Steam sterilization is a critical process in medical and pharmaceutical settings. Clean steam can be used to sterilize equipment within a controlled environment. The steam sterilization process is commonly used in the following applications:
- Biopharmaceutical manufacturing: In these settings, bacteria, animal cells, or yeasts are grown to produce consistent biopharmaceutical components. A sterile, controlled environment is crucial.
- Manufacturing sterile solutions, such as injectable and parenteral solutions, ophthalmic products, and more.
To provide sterilization, clean steam is injected into any of these three containment types:
- Piping: (CIP) Steam can clean and sterilize piping that is used to transport or measure fluid components to eliminate buildup.
- Autoclaves: Equipment, devices, containers, and other components or equipment are loaded into an autoclave.
- Equipment: Some equipment and enclosed spaces are built to have clean steam directly pumped into its interior.

Benefits of Clean Steam for the Pharmaceutical Industry
Clean steam makes maintaining a clean, sterile environment simple. By controlling the temperature and composition of the steam, facilities can sterilize a multitude of goods that need a consistent, no-contact clean but which may have edges, complex geometries, and small interiors that make traditional sterilization processes impossible. Some of the key benefits of using clean steam in the pharmaceutical industry include:
Reduced Corrosion Potential
Traditional cleaning processes can increase the risk of corrosion in metal parts because liquids can get trapped or process buildup may linger. Traditional utility steam by itself is known to cause corrosion, so it must be paired with corrosion-inhibiting additives to the steam. Clean steam avoids this risk, which allows sensitive equipment to operate with less risk of corrosion. This, in turn, results in higher-purity products and lower operating costs because the machinery has a longer lifespan.
Prevention of Microbial Growth
Clean steam is a high-temperature steam that can kill organic pathogens and microorganisms that would otherwise put pharmaceutical processes at risk of contamination. Clean steam generators are built to eliminate the buildup of condensation while dangerous contaminants can linger.
Preventing Entry of Contaminants
Clean steam is sterile at the point of vaporization, but traditional steam generators and tools have multiple vulnerable points where chemical and biological contamination can enter the steam. Clean steam equipment is built to keep the steam pure throughout the entire sterilization process.
Clean Steam Units from Electro Steam
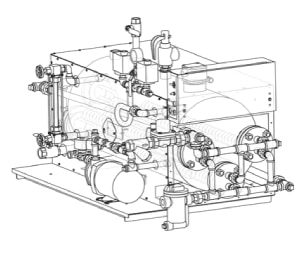
Electro-Steam Generator Corp. is an American-made OEM manufacturer that has provided high quality electric clean steam generators since 1952. Our “bench top” LG Series to our larger, more powerful LB Series or any unit custom unit are designed to suit any application. Our high quality, highly efficient Clean Steam units are 316L Stainless Steel on all wetted surfaces with most units available in indirect steam to steam models if your facility has utility steam in place.
All Electro-Steam generators feature adjustable pressure controls, safety valves, and automatic low water cutoffs. Integral units have Stainless Steel electrical boxes and stand-alone models have complete Stainless Steel cabinets. Contact us today to learn more about our offerings and discuss your cleans steam needs.
Added benefits include:
- Built by an ASME Certificate holder in accordance with ASME BPVC Section I – Rules for Construction of Power Boilers (“ASME BPVC Section I”). Compliance with the requirements outlined in The National Board Synopsis of Boiler and Pressure Vessel Laws, Rules and Regulations (NB-370) RULES FOR CONSTRUCTION AND STAMPING section, which for many jurisdictions include but are not limited to ASME BPVC Section I, ASME CSD-1, ASME B31.1, and REGISTRATION WITH THE NATIONAL BOARD
- UL and cUL (Canada) listed
- 99% efficient resulting in energy and water conservation
- Low or high pressure steam in less than 15 minutes (or on demand)
- A one year warranty on all parts and a five year warranty on the chamber
To learn more about our Clean Steam units or to request a quote, please see the table below.
| Models | |||

|
S.S. LG-Series | 10, 15, 20, 25, 30 and 40 kW | test |

|
S.S LB-Series | 40, 50, 60, 80, 100, 120, 150, 180, and 240-480 kW | |

|
Vblock Series | 10, 15, 20, 25, 30, 40 | |
|
Indirect Steam Series | Indirect Clean Steam Generator |
 Proudly Manufactured in the USA
Proudly Manufactured in the USA